The History of The Atomic Model
The atom is the smallest unit of matter that makes up everything around us. Different types of atoms form the elements of the periodic table. While each element has its own unique properties, every atom contains the same subatomic particles: protons, neutrons, and electrons.
However, it took hundreds of years of careful observation, experimentation, and revision for scientists to figure out what atoms are made of and how they are arranged. Today, our most accurate description is called the quantum model of the atom.
Let’s explore the history of the atomic model and some of the scientists who helped shape our current understanding.
Democritus, 450 BC
Democritus was a Greek philosopher. Long before scientific labs existed, philosophers tried to explain the nature of matter. Aristotle believed you could divide a grain of sand infinitely. Democritus disagreed, arguing that there must be a smallest piece of matter that could not be divided further.
He called this piece the atomos, meaning “indivisible” in Greek. While Democritus had no way to prove his idea and Aristotle’s views were more widely accepted, his concept of atoms laid the foundation for the scientific discoveries that came much later.
John Dalton, 1804
John Dalton, an English schoolteacher and chemist, revived the idea of atoms with one of the first true atomic theories. By studying the way elements combined in fixed ratios (the law of definite proportions) and connecting it with the law of conservation of mass, he proposed:
All matter is made of atoms.
Atoms of the same element are identical.
Atoms cannot be created, divided, or destroyed.
Atoms of different elements can combine in simple ratios to form compounds.
In chemical reactions, atoms are rearranged.
Dalton pictured atoms as solid spheres, like tiny billiard balls, with no internal structure. This became known as the solid sphere model.
We now know that parts of Dalton’s theory are not entirely correct. Atoms can be subdivided (into protons, neutrons, and electrons), isotopes show that atoms of the same element can have different masses, and nuclear reactions can change atoms into other elements. But Dalton’s work was a crucial first step in building a scientific model of the atom.
Michael Faraday, 1830s
Michael Faraday, an English scientist, studied the relationship between electricity and matter. Through his experiments on electrolysis (using electricity to break down compounds), Faraday discovered that atoms and electricity were connected. He showed that electrical forces play a role in holding compounds together and even coined words we still use today: electrode, cathode, anode, and ion.
Faraday did not discover subatomic particles, but his work suggested that atoms themselves must contain electrical components. This insight laid the groundwork for experiments with cathode rays later in the 1800s, which eventually revealed the electron.
Cathode Ray Tubes
After Faraday’s work suggested that electricity was connected to atoms, scientists began experimenting with devices called cathode ray tubes. A cathode ray tube is a sealed glass tube with almost all of the air removed. When electricity is passed through it, a glowing beam travels from the cathode (negative end) to the anode (positive end).
At first, no one knew what this mysterious “ray” was. But experiments showed that the beam could cast shadows, move small paddles, and bend when magnets or electric fields were nearby. This meant that the rays had both mass and charge.
These cathode rays would become the focus of J. J. Thomson’s experiments. By studying them more closely, he discovered that they were actually tiny, negatively charged particles later named electrons — the first subatomic particle ever identified.
Image Credit: Figure 2.6. Thomson’s cathode-ray experiments. Adapted from Chemistry 2e: 2.2 Evolution of atomic theory (OpenStax, 2019), https://openstax.org/books/chemistry-2e/pages/2-2-evolution-of-atomic-theory, CC BY 4.0. Image credits: modification of work by Nobel Foundation; modification of work by Eugen Nesper; modification of work by Kurzon/Wikimedia Commons.
J. J. Thomson, 1897
Using a cathode ray tube, J. J. Thomson discovered the electron, the first identified subatomic particle. He showed that the mysterious rays inside the tube were actually tiny negatively charged particles that were much smaller than atoms.
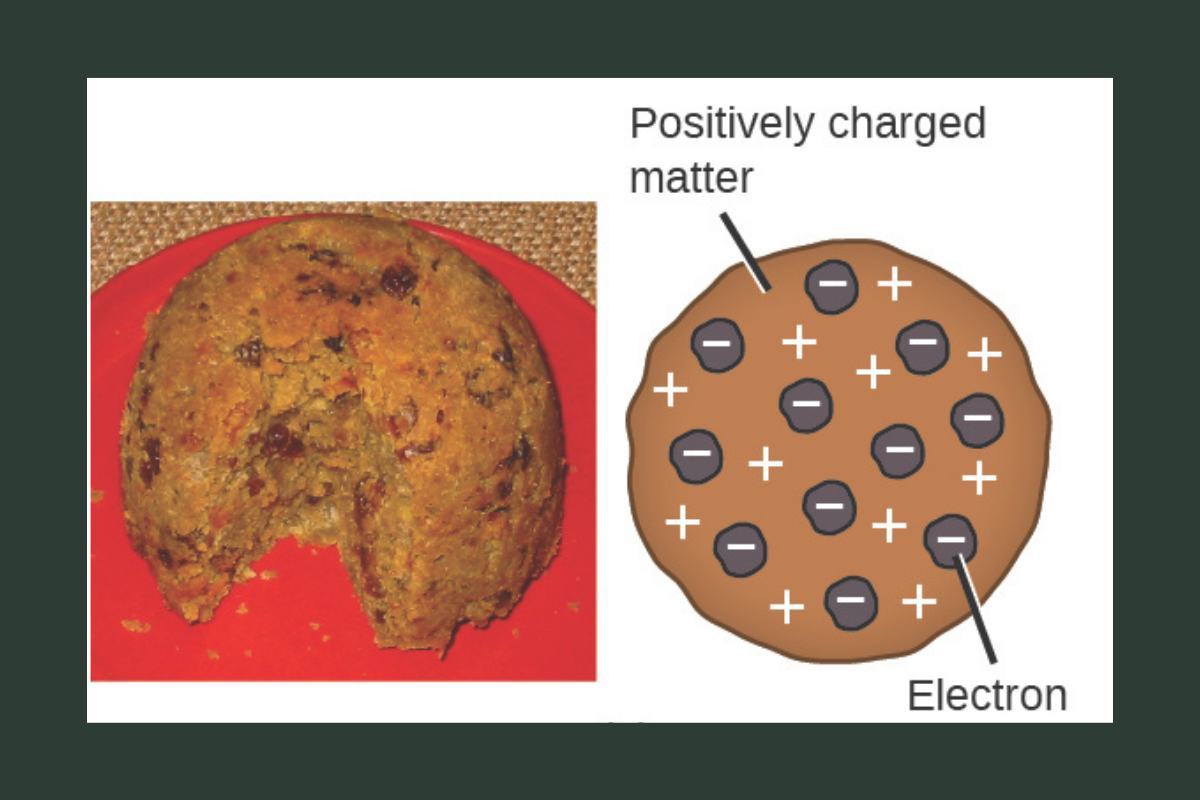
Thomson proposed the plum pudding model of the atom. He imagined atoms as positively charged spheres with negative electrons scattered throughout, like raisins in pudding. While later experiments showed this model was incorrect, it was the first to show that atoms were not indivisible.
Image Credit: Figure 2.8. Early atomic models: (a) Thomson’s “plum pudding” model; (b) Nagaoka’s Saturn-like model. Adapted from Chemistry 2e: 2.2 Evolution of atomic theory (OpenStax, 2019), https://openstax.org/books/chemistry-2e/pages/2-2-evolution-of-atomic-theory, CC BY 4.0. Image credits: modification of work by Man vyi/Wikimedia Commons; modification of work by NASA/Wikimedia Commons.
Robert Millikan, 1909
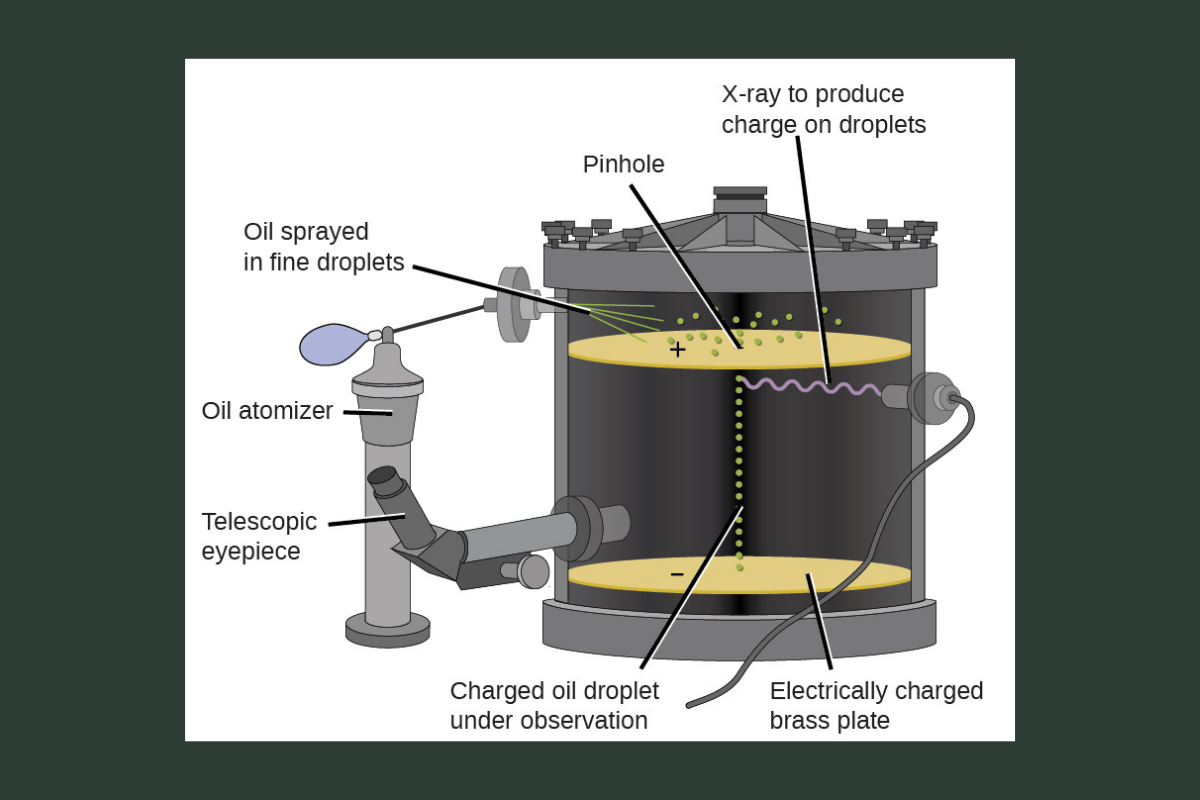
Robert Millikan, an American physicist, measured the charge of the electron with his famous oil drop experiment. By balancing the downward pull of gravity with the upward electric force on tiny charged oil droplets, Millikan determined the exact charge of a single electron. This also allowed scientists to calculate its mass.
Image Credit: Figure 2.7. Millikan’s oil-drop experiment. Adapted from Chemistry 2e: 2.2 Evolution of atomic theory (OpenStax, 2019), https://openstax.org/books/chemistry-2e/pages/2-2-evolution-of-atomic-theory, CC BY 4.0.
Ernest Rutherford, 1911
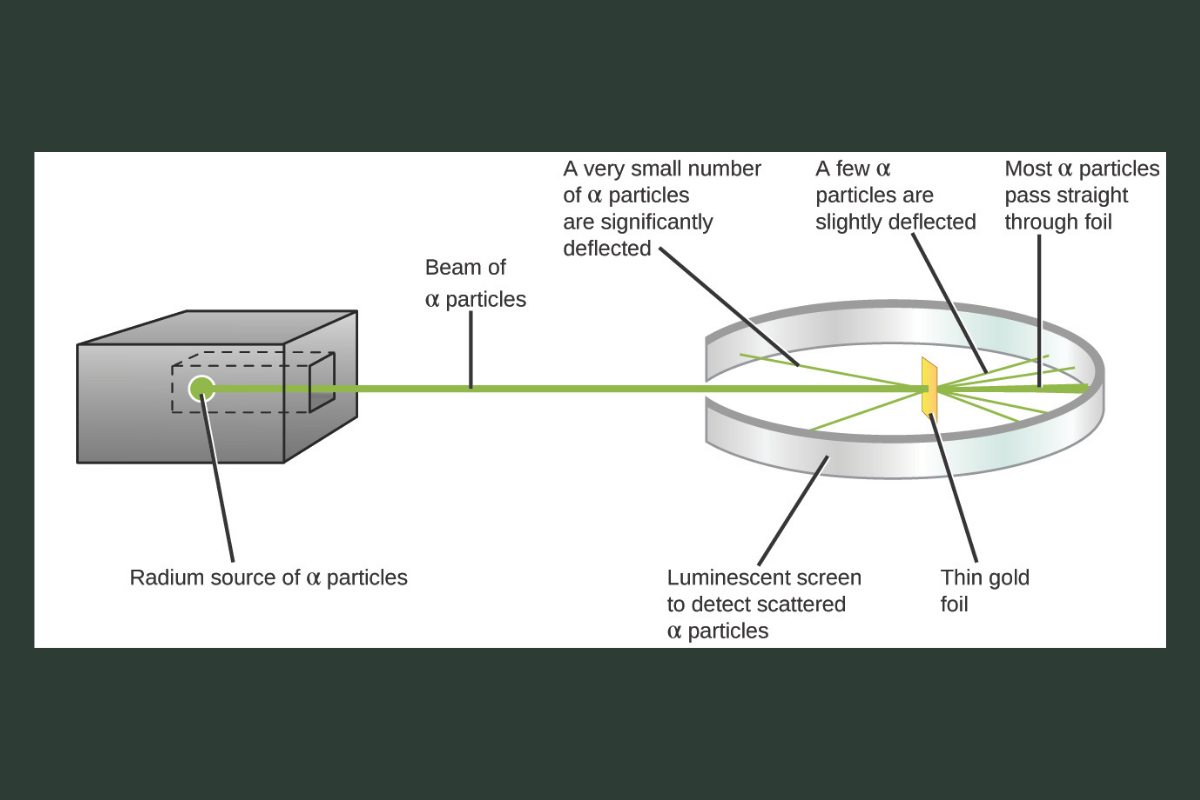
Ernest Rutherford, often called the “father of nuclear physics,” performed the gold foil experiment. He shot positively charged alpha particles at a thin sheet of gold foil. Most particles passed straight through, but a few bounced back at sharp angles.
This surprising result showed that atoms are mostly empty space, with a small, dense, positively charged center called the nucleus. Rutherford’s model introduced the idea that electrons orbit this central nucleus, similar to planets around the sun.
Image Credit: Figure 2.9. Rutherford’s gold-foil experiment. Adapted from Chemistry 2e: 2.2 Evolution of atomic theory (OpenStax, 2019), https://openstax.org/books/chemistry-2e/pages/2-2-evolution-of-atomic-theory, CC BY 4.0.
Henry Moseley, 1913
Henry Moseley, an English physicist, studied X-rays emitted by different elements. He discovered that each element has a unique number of protons in its nucleus, now known as the atomic number.
Moseley’s work corrected the arrangement of the periodic table, proving that elements should be ordered by atomic number rather than atomic mass.
Niels Bohr, 1913
Niels Bohr improved Rutherford’s model by applying new ideas from quantum theory. He suggested that electrons travel in fixed energy levels (or shells) around the nucleus, and that they can jump between levels by absorbing or releasing energy.
This became known as the Bohr Model or planetary model of the atom. It successfully explained the spectral lines of hydrogen, or the colors emitted by hydrogen. This was later replaced by more advanced models for larger atoms.
Louis de Broglie and Erwin Schrödinger, 1920s
Louis de Broglie proposed that electrons have a dual nature — they act like both particles and waves. Building on this idea, Erwin Schrödinger developed mathematical equations to describe where electrons are most likely to be found.
This became the quantum mechanical model of the atom. In this model, electrons are not pictured as moving in fixed orbits, but as existing in regions of probability called orbitals.
📦 Fun Fact: Schrödinger’s Cat
In 1935, Erwin Schrödinger imagined a strange scenario to explain how weird quantum physics can be. He described a cat in a box with a device triggered by the decay of a radioactive atom. Since the atom could both decay and not decay at the same time (a quantum principle called superposition), the cat could be thought of as both alive and dead at once—at least until someone opened the box.
This thought experiment wasn’t about atoms directly, but it highlighted the same quantum ideas Schrödinger used in his wave model of the atom.
James Chadwick, 1932
James Chadwick discovered the neutron, an uncharged particle located in the nucleus alongside protons. Neutrons explained why atoms of the same element can have different masses (isotopes).
With the discovery of neutrons, the modern picture of the atom — protons and neutrons in the nucleus with electrons in surrounding orbitals — was complete.